Milestone-Proposal:Laser Ionization Mass Spectrometer, 1988
To see comments, or add a comment to this discussion, click here.
Docket #:2023-29
This Proposal has been approved, and is now a Milestone
To the proposer’s knowledge, is this achievement subject to litigation? No
Is the achievement you are proposing more than 25 years old? Yes
Is the achievement you are proposing within IEEE’s designated fields as defined by IEEE Bylaw I-104.11, namely: Engineering, Computer Sciences and Information Technology, Physical Sciences, Biological and Medical Sciences, Mathematics, Technical Communications, Education, Management, and Law and Policy. Yes
Did the achievement provide a meaningful benefit for humanity? Yes
Was it of at least regional importance? Yes
Has an IEEE Organizational Unit agreed to pay for the milestone plaque(s)? Yes
Has the IEEE Section(s) in which the plaque(s) will be located agreed to arrange the dedication ceremony? Yes
Has the IEEE Section in which the milestone is located agreed to take responsibility for the plaque after it is dedicated? Yes
Has the owner of the site agreed to have it designated as an IEEE Milestone? Yes
Year or range of years in which the achievement occurred:
1988
Title of the proposed milestone:
Laser Ionization Mass Spectrometer, 1988
Plaque citation summarizing the achievement and its significance; if personal name(s) are included, such name(s) must follow the achievement itself in the citation wording: Text absolutely limited by plaque dimensions to 70 words; 60 is preferable for aesthetic reasons.
In 1988, Shimadzu Corporation released a mass spectrometer that could measure macromolecules whose molar mass was at least 50,000 grams per mole. As the world's first commercially available device that applied soft laser desorption ionization techniques, it led to new pharmaceuticals and diagnostic capabilities in the fields of molecular biology and medicine. Koichi Tanaka, the key developer of this technology, shared the 2002 Nobel Prize in Chemistry.
200-250 word abstract describing the significance of the technical achievement being proposed, the person(s) involved, historical context, humanitarian and social impact, as well as any possible controversies the advocate might need to review.
This Milestone Proposal is about mass spectrometers for proteins, which are macromolecules. In the 1980s, mass spectrometry of macromolecular proteins became necessary in various fields such as molecular biology, medicine, and pharmacology. In general, molecules are fragile, and it was not possible to measure the mass of large molecules. First of all, in 1985, Shimadzu became the first company in the world to succeed in ionizing proteins without destroying them, which had been considered impossible until then Next, we developed a technology called "inclined electrolytic ion reflector" and "delayed ion extraction" in a device equipped with this technology to improve mass resolution. In addition, innovations such as high sensitivity that accelerates ions immediately before the detector, blind ion-electron converters that extend the life of the detector, and high-speed, high-precision spectral integrated circuits have been incorporated. In 1989, the development of this technology led to mass spectrometry of macromolecules with a molecular weight of more than 70k molecules. Shimadzu was the first commercially available product in the world to realize this technology with the LAMS-50K, which was released by Shimadzu Corporation in 1988. Koichi Tanaka of Shimadzu Corporation was awarded the Nobel Prize in Chemistry in 2002 for his development of a soft laser desorption/ionization method for mass spectrometry of biopolymers. Products based on this technology have since been developed by many researchers, and are now indispensable in various fields such as molecular biology, medicine, and pharmacology.
IEEE technical societies and technical councils within whose fields of interest the Milestone proposal resides.
IEEE Consumer Technology Society
IEEE Engineering in Medicine & Biology Society
In what IEEE section(s) does it reside?
IEEE Kansai Section
IEEE Organizational Unit(s) which have agreed to sponsor the Milestone:
IEEE Organizational Unit(s) paying for milestone plaque(s):
Unit: IEEE Kansai Section
Senior Officer Name: Yoshinobu Kajikawa
IEEE Organizational Unit(s) arranging the dedication ceremony:
Unit: IEEE Kansai Section
Senior Officer Name: Yoshinobu Kajikawa
IEEE section(s) monitoring the plaque(s):
IEEE Section: IEEE Kansai Section
IEEE Section Chair name: Yoshinobu Kajikawa
Milestone proposer(s):
Proposer name: Yoshinari Shirasaki
Proposer email: Proposer's email masked to public
Please note: your email address and contact information will be masked on the website for privacy reasons. Only IEEE History Center Staff will be able to view the email address.
Street address(es) and GPS coordinates in decimal form of the intended milestone plaque site(s):
1, Nishinokyo Kuwabara-cho, Nakagyo-ku, Kyoto 604-8511, Japan 35.00951843094894, 135.72865759059437
Describe briefly the intended site(s) of the milestone plaque(s). The intended site(s) must have a direct connection with the achievement (e.g. where developed, invented, tested, demonstrated, installed, or operated, etc.). A museum where a device or example of the technology is displayed, or the university where the inventor studied, are not, in themselves, sufficient connection for a milestone plaque.
Please give the details of the mounting, i.e. on the outside of the building, in the ground floor entrance hall, on a plinth on the grounds, etc. If visitors to the plaque site will need to go through security, or make an appointment, please give the contact information visitors will need. a corporate building
Are the original buildings extant?
Yes
Details of the plaque mounting:
a corner of a showroom in the building
How is the site protected/secured, and in what ways is it accessible to the public?
The manager of Shimadzu Corporation oversees the protection of the showroom. Guests who apply and receive permission from the following URL can tour the showroom and access this plaque: https://www.shimadzu.com/form/ci/contact.html
Who is the present owner of the site(s)?
Shimadzu Corporation
What is the historical significance of the work (its technological, scientific, or social importance)? If personal names are included in citation, include detailed support at the end of this section preceded by "Justification for Inclusion of Name(s)". (see section 6 of Milestone Guidelines)
Justification of Name in the Citation
It is essential to include the name of Kōichi Tanaka in the citation. He is the inventor of the foundational technology, the ‘Soft Laser Desorption/Ionization’ technology, which enabled the realization of this innovative mass spectrometer. Due to his significant contributions to humanity through this groundbreaking technology, he was awarded the Nobel Prize in 2002 [3][4]. This mass spectrometer represents the first commercially available product built upon this technology. By acknowledging his prestigious award in the citation, the profound significance of the birth of this device is fully expressed.
As described in references [3][4], the development of this instrument was achieved through the collaboration of multiple engineers at Shimadzu Corporation. Tamio Yoshida and Yoshikazu Yoshida developed the high-resolution mass analysis spectrometer, Yutaka Ido developed the detector for macromolecules, and Satoshi Akita developed the high-speed signal processing circuit. By combining these specially developed technologies, this practical instrument was born, and it was demonstrated that Tanaka's invention is revolutionarily useful and valuable.
The Nobel Prize in Chemistry 2002 was awarded "for the development of methods for identification and structure analyses of biological macromolecules" with one half jointly to John B. Fenn and Koichi Tanaka "for their development of soft desorption ionisation methods for mass spectrometric analyses of biological macromolecules" and the other half to Kurt Wüthrich "for his development of nuclear magnetic resonance spectroscopy for determining the three-dimensional structure of biological macromolecules in solution"[18]. Fenn and Tanaka developed electrospray ionization (ESI) and soft laser desorption ionization (SLD) respectively and applied them to protein mass spectrometry. Mass spectrometry is method to determine molecular weight of compounds and is fundamentally different from NMR in principle. The SLD method led to matrix-assisted laser desorption/ionization (MALDI) mass spectrometry [3],[12],[13],[14],[15],[16]. MALDI mass spectrometer became widely adopted worldwide. This proposal is for the first commercially available MALDI mass spectrometer developed by Tanaka et al. On the other hand, the ESI method is a technology that ionizes droplets by applying an electric field[19]. Due to its characteristics, it became closely associated with liquid chromatography (LC), which primarily handles solution samples, leading to significant development as LC/MS.
Historical significance of the work
Background
This Milestone Proposal is about mass spectrometers for proteins, which are macromolecules. In the 1980s, mass spectrometry of macromolecular proteins became necessary in various fields such as biology, medicine, and pharmacology. In general, ionization of macromolecules without decomposition is difficult, so it was not possible to measure the mass of large molecules.
Technical and Scientific importance
Shimadzu planned to develop and commercialize a mass spectrometer for biomolecules such as proteins. Tanaka et al. developed a time-of-flight mass spectrometer for this purpose.
Firstly, in 1985, Shimadzu succeeded for the first time in the world in "ionizing proteins without decomposing them", which had been considered to be impossible until then [1]. This paved the way for the study of proteins by mass spectrometry. Next, they developed a new technology called "inclined field ion reflector" to improve mass resolution. In addition, innovations such as high sensitivity that accelerates ions in front of the detector, ion-electron converters that extend the life of the detector, and high-speed, high-precision spectral accumulation circuits have been incorporated. Finally, in 1988, the development of these technologies led to mass spectrometry of macromolecules with a molecular weight of more than 50,000.
The world's first commercially available product to realize this technology was Shimadzu Corporation's LAMS-50K released in 1988 [2], [3], [4]. The device was delivered to the 'City of Hope's Beckman Research Institute' in the United States in 1990 [5].
In recognition of this achievement, Dr. Tanaka of Shimadzu Corporation was awarded the Nobel Prize in Chemistry in 2002 “for their development of soft desorption ionisation methods for mass spectrometric analyses of biological macromolecules” [3].
Social importance
Products based on this technology have since been developed by many researchers, and are now indispensable in various fields such as biology, medicine, and pharmacology. Applied development of this method is still actively carried out, and 1,375 units of equipment using this method categorized as 'MALDI TOF instruments' were sold in 2022. The market is projected to grow with a CAGR of 5.7% from 2022 to 2027 [6]. A prominent use case in recent years is microbial identification [7]. In addition, mass microscopes have been developed using this method [8].
What obstacles (technical, political, geographic) needed to be overcome?
Innovative Technologies for obstacles
Technical obstacles
Proteins are molecules playing important role in life phenomena. Molecular mass determination is the most fundamental basis for this analysis, and it was hoped that it could be realized by mass spectrometry. However, mass spectrometry requires ionization of the molecule of interest. Until the mid-1980s, it was impossible to ionize proteins, which are large molecules with a molecular weight of 10,000 or more, without decomposing them. Methods such as Field Desorption (FD) [9] and Fast Atom Bombardment (FAB) [10], which were ionization methods developed at that time, decomposed large molecules such as proteins. Therefore, a method of ionizing macromolecules without decomposing them has been eagerly awaited [3].
Mass Spectrometry
Mass spectrometry is an analytical technique that is used to measure the mass of molecules. The mass of molecules is almost the same as the molecular weight (MW). For example, using mass spectrometry, the components of air [e.g., nitrogen (N₂, MW=28) and oxygen (O₂, MW=32)] would be separated according to their mass. Mass spectrometry allows us to determine the mass of molecules; thus, if we detect molecules with the masses of 28 and 32 in air sample, we can easily infer that these masses correspond to N₂ and O₂. In such way, mass spectrometry is an excellent technique for analyzing substances, which allows identification of components in a sample by measuring the masses of molecules.
Methods of mass spectrometry
Mass spectrometry consists of three basic steps: “ionization”, “mass separation”, and “detection”.
Ionization is a process by which molecules become charged (charged molecules are called ions). The generated ions have a physical quantity called the mass-to-charge ratio, i.e. the mass of the ion divided by its charge. During mass separation, ions can be separated according to mass-to-charge ratio in a vacuum vessel by electric or magnetic fields [11]. Finally, those separated ions arrive at detector and are observed as individual signals.
Electric or magnetic fields affect the motion of ions in the vacuum because the ions have electric charges. The ions are accelerated by applying a voltage and fly in a straight line. As the ions subsequently pass through the magnetic field, they are forced by the magnetic field to bend their orbits. The degree of bending depends on the mass-to-charge ratio of the ion. In other words, by precisely setting the electric field or magnetic field, it is possible to determine mass-to-charge ratio of ions of interest or to detect the ions of a specific mass-to-charge ratio.
Mass spectrometer
In 1988, the world's first mass spectrometer capable of measuring biopolymers, the LAMS-50K, was launched [4], (Figure 1).
Koichi Tanaka was involved in the development of the system, and it was the starting point of the foundation of the current mass spectrometer. Figure 2 show the "sample holder and ion extractor" of the LAMS-50K. This component has been improved and are currently used in our mass spectrometers. The parts in the photo are the same as those used for the LAMS-50K.
In order to reach this point in terms of the shape and material of these parts, various knowledge and skills such as physics, chemistry, biology, materials engineering, electrical engineering, and mechanical engineering are required. The "sample holder and ion extractor" is permanently displayed at the Shimadzu Founding Memorial Museum in Kyoto, Japan.
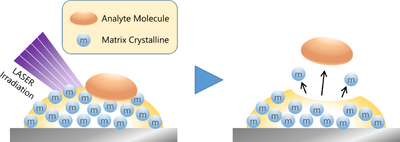
Matrix in mass spectrometry
The matrix is described as the key to MALDI (Matrix-Assisted Laser Desorption/Ionization) [12], one of the ionization methods used in mass spectrometry.
The concept of MALDI was coined by Hillenkamp et al. in 1985. While ionizing alanine mixed with tryptophan, two amino acids, with ultraviolet irradiation, they found an increase in the ionization efficiency of alanine. Alanine itself does not absorb UV light, but tryptophan, the surrounding environment (matrix), does. Hillenkamp et al. explained that tryptophan helped (or assisted) the ionization of alanine by receiving the ultraviolet light energy, and they called this mechanism “Matrix-Assisted Laser Desorption.” Thus, tryptophan was the world's first MALDI matrix.
This MALDI technology, along with the Soft Laser Desorption (SLD) ionization method that was developed by Tanaka et al. at the same time, which realized the ionization of proteins [13],[14],[15], has undergone rapid development and become an indispensable measurement technology in proteomics research, which was expanding at that time. It is still widely used in combination with Electrospray Ionization (ESI), which is an ionization method based on a completely different principle developed by Fenn at approximately the same time, and continues to evolve.
As is clear from the MALDI schematic (Figure 3), the key to a MALDI measurement is the matrix. Because the optimal matrix and protocol differ depending on the substance to be measured, it is essential to investigate precedent cases in advance.
What features set this work apart from similar achievements?
Soft Laser Desorption / Ionization technology
Shimadzu Corporation released a mass spectrometer that can measure macromolecules of molecular weight extended to 50,000 in 1988. This device is the world's first commercially available device that applies Soft Laser Desorption / Ionization technology. Koichi Tanaka, who developed this technology, won the 2002 Nobel Prize in Chemistry [3],[4]. With the spread of this type of device, various fields such as biology and medicine have developed. This technology is a breakthrough invention recognized by the Nobel Prize Committee in 2002 and cannot be compared to other similar technologies such as Field Desorption(FD) [9] or Fast Atom Bombardment(FAB) [10]. Before the Soft Laser Desorption/Ionization technology developed, a primitive approach "Laser Ionization" was tried since 1960's [17] and commercial equipments (e.g. LAMMA 1000, Leybold-Heraeus, Germany, 1983) exist, but could not be applied to macromolecule analysis.
In order to realize the world's first commercially available mass spectrometer that applies the Soft Laser Desorption/Ionization technology, Shimadzu engineers developed and implemented various new technologies and succeeded in launching it [2][3][4][13][14].
Koichi Tanaka received the Nobel Prize in Chemistry 2002
Mass spectrometry originated in England about 100 years ago. In a mass spectrometer used at that time, neon (a noble gas) was ionized by electric discharge in a vacuum vessel. Generated ions were accelerated by the electric potential. Then, ionized neon atoms were separated into ²⁰Ne (mass=20) and its isotope ²²Ne (mass=22) by bending their trajectories by magnetic field. Finally, these ions were detected on a photographic plate. Currently, mass spectrometers are more sophisticated than they originally were. However, these instruments still consist of three basic components.
In 2002, John B. Fenn and Koichi Tanaka won the Nobel Prize in Chemistry “for the development of methods for identification and structure analyses of biological macromolecules[18]. ” In the 1980s, Fenn and Tanaka developed electrospray ionization (ESI) and soft laser desorption ionization (SLD), respectively, which could be applied to protein analysis. In 1988, Dr. Karas et al. published a paper on the successful ionization of proteins with a molecular weight over 10,000 [16]. However, the Nobel Prize committee confirmed that Tanaka was the true pioneer [1],[3],[13]. Tanaka's innovative technology led to matrix-assisted laser desorption/ionization (MALDI) mass spectrometry [3],[12],[13],[14],[15],[16].
On the other hand, the ESI method, introduced by Fenn in 1988, is a technology that ionizes droplets by applying an electric field[19]. Due to its characteristics, it became closely associated with liquid chromatography (LC), which primarily handles solution samples, leading to significant development as LC/MS.
These two kinds of mass spectrometer, based on completely different ionization methods in principle, which were independently developed, have each undergone their own evolution and have gained widespread adoption, leveraging their respective merits. Therefore, it can be said that this laser ionization mass spectrometer developed by Tanaka et al. was created, developed, and made contributions to society completely independently from Fenn's invention.
Mass spectrometer is an indispensable tool for comprehensive protein analysis so-called proteomics, which is widely used in biomarker research. Such analysis would not be possible without the advent of ionization technologies such as ESI and MALDI. Innovative ionization technologies have the potential to considerably expand the scope of analytical science.
Why was the achievement successful and impactful?
Supporting texts and citations to establish the dates, location, and importance of the achievement: Minimum of five (5), but as many as needed to support the milestone, such as patents, contemporary newspaper articles, journal articles, or chapters in scholarly books. 'Scholarly' is defined as peer-reviewed, with references, and published. You must supply the texts or excerpts themselves, not just the references. At least one of the references must be from a scholarly book or journal article. All supporting materials must be in English, or accompanied by an English translation.
References
[1] T. Yoshida and K. Tanaka, "Sample preparation method and sample holder for laser ionization mass spectrometer," (in Japanese), Japan Patent JP01769145, Feb. 2, 1987.
[Note] The patent public number is ”特開昭62-043562” and the title is "レーザイオン化質量分析計用試料作成方法および試料ホルダ” in Japanese.
[2] "Shimadzu Corporation releases laser ionization mass spectrometer Compatible with a mass number of 50,000," (in Japanese), Nikkan Kogyo Shimbun, p.18, Feb. 15,1988.
[Note] Nikkan Kogyo Shimbun (日刊工業新聞, The Daily Industrial News), one of the leading daily newspapers in Japan, specializes in business and industrial affairs, and is published by The Nikkan Kogyo Shimbun, Ltd. Circulation is around 420,000. The newspaper was inaugurated in 1915.
[3] K. Tanaka, ”The Origin of Macromolecule Ionization by Laser Irradiation (Nobel Lecture),“ Angew. Chem. Int. Ed. Engl., vo. 42, pp. 3861-3870, 2003, doi: 10.1002/anie.200300585
[Note] It states that the product was sold to the City of Hope's Beckman Research Institute (USA). There is a description of the address of Tanaka (= Shimadzu Corporation). Key points of the equipment configuration, including award-winning technology, are also described. No product model number is listed on this reference, but product model number can be seen on below reference [4].
[4] Nobel Prize Outreach , “Koichi Tanaka Facts,” Accessed: Jan. 29, 2024. [Online]. Available: https://www.nobelprize.org/prizes/chemistry/2002/tanaka/facts/
[Note] On the Nobel Committee's website, it is stated that Shimadzu's product "LAMS-50K was released in 1988".
[5] Beckman Research Institute of City of Hope, “CITY OF HOPE CITY OF TOMMOROWS,” p. 5, 1990.
[Note] Annual report of the institute about 1990. Koichi Tanaka and LAMS-50K is shown in the photo.
[6] Strategic Directions International, “The Laboratory Analytical and Life Science Instrumentation Industry,” Global Assessment Report 2023, p. 260, 2023.
[7] P. A. Demirev, Y-P. Ho, V. Ryzhov, and C. Fenselau, ”Microorganism Identification by Mass Spectrometry and Protein Database Searches,” Anal. Chem., vol. 71, no. 14, pp. 2732-2738, 1999, doi: 10.1021/ac990165u.
[8] R. M. Caprioli, T. B. Farmer, and J. Gile, "Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS," Anal. Chem., vol. 69, no. 23, pp. 4751-4760, 1997, doi: 10.1021/ac970888i.
[9] H. D. Beckey, "Field desorption mass spectrometry: A technique for the study of thermally unstable substances of low volatility," Int. J. Mass Spectrom. And Ion Phys., vol. 2, issue 6, pp. 500-502, Jun.-Jul. 1969, doi: 10.1016/0020-7381(69)80047-1.
[10] M. Barber, R. S. Bordoli, R. D. Sedgwick, and A. N. Tyler, "Fast atom bombardment of solids as an ion source in mass spectrometry," Nature, vo. 293, pp. 270-275, 1981, doi: 10.1038/293270a0
[11] J. J. Thomson, "On Rays of Positive Electricity", Phil. Mag. And J. Sci., series 6, pp. 561-575, May 1907.
[12] F. Hillenkamp, M. Karas, R. C. Beavis, and B. T. Chait, “Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry of Biopolymers”, Anal. Chem., vol. 63, issue 24, pp. 1193A-1203A, 1991, doi: 10.1021/ac00024a716.
[13] K. Tanaka, Y. Ido, S. Akita, Y. Yoshida, and T. Yoshida, "Detection of High Molecules by Laser Desorption Time-of flight Mass Spectrometry," Second Japan-China Joint Symposium on Mass Spectrometry, pp. 185-188, 1987.
[Note] You can view it at the following URL: https://masspec.scripps.edu/learn/ms/pdf/1988_Tanaka.pdf
[14] K. Tanaka, H. Waki, Y. Ido, S. Akita, Y. Yoshida, T. Yoshida, and T. Matsuo, "Protein and Polymer Analyses up to m/z 100000 by Laser Ionization Time-of flight Mass Spectrometry," Rapid Comm. Mass Spectrom., vol. 2, issue 8, pp. 151-153, 1988, doi: 10.1002/rcm.1290020802.
[15] R. J. Cotter, "Time-of-Flight Mass Spectrometry" (ACS Professional Reference Book), p. 128, 1997.
[16] M. Karas, F. Hillenkamp, “Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons,” Anal. Chem., vol. 60, issue 20, pp. 2299–2301, 1988, doi: 10.1021/ac00171a028
[17] R. E. Honig and J. R. Woolston, "LASER‐INDUCED EMISSION OF ELECTRONS, IONS, AND NEUTRAL ATOMS FROM SOLID SURFACES," Appl. Phys. Lett., volume 2, pp. 138-139, 1963, doi: 10.1063/1.1753812
[18] The Nobel Prize in Chemistry 2002. NobelPrize.org. Nobel Prize Outreach AB 2024. Accessed: Mar. 5, 2024. [Online]. Available: https://www.nobelprize.org/prizes/chemistry/2002/summary/
[19] J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong, C. M. Whitehouse, “Electrospray Ionization for Mass Spectrometry of Large Biomolecules,” Science, vol. 246, issue 4926, pp. 64–71, 1989, doi:10.1126/science.2675315
Supporting materials (supported formats: GIF, JPEG, PNG, PDF, DOC): All supporting materials must be in English, or if not in English, accompanied by an English translation. You must supply the texts or excerpts themselves, not just the references. For documents that are copyright-encumbered, or which you do not have rights to post, email the documents themselves to ieee-history@ieee.org. Please see the Milestone Program Guidelines for more information.
Please email a jpeg or PDF a letter in English, or with English translation, from the site owner(s) giving permission to place IEEE milestone plaque on the property, and a letter (or forwarded email) from the appropriate Section Chair supporting the Milestone application to ieee-history@ieee.org with the subject line "Attention: Milestone Administrator." Note that there are multiple texts of the letter depending on whether an IEEE organizational unit other than the section will be paying for the plaque(s).
Please recommend reviewers by emailing their names and email addresses to ieee-history@ieee.org. Please include the docket number and brief title of your proposal in the subject line of all emails.