Milestone-Proposal:First Atomic Clock
To see comments, or add a comment to this discussion, click here.
Docket #:2016-05
This Proposal has been approved, and is now a Milestone
To the proposer’s knowledge, is this achievement subject to litigation? No
Is the achievement you are proposing more than 25 years old? Yes
Is the achievement you are proposing within IEEE’s designated fields as defined by IEEE Bylaw I-104.11, namely: Engineering, Computer Sciences and Information Technology, Physical Sciences, Biological and Medical Sciences, Mathematics, Technical Communications, Education, Management, and Law and Policy. Yes
Did the achievement provide a meaningful benefit for humanity? Yes
Was it of at least regional importance? Yes
Has an IEEE Organizational Unit agreed to pay for the milestone plaque(s)? Yes
Has the IEEE Section(s) in which the plaque(s) will be located agreed to arrange the dedication ceremony? Yes
Has the IEEE Section in which the milestone is located agreed to take responsibility for the plaque after it is dedicated? Yes
Has the owner of the site agreed to have it designated as an IEEE Milestone? Yes
Year or range of years in which the achievement occurred:
1947-1949
Title of the proposed milestone:
First Atomic Clock, 1948
Plaque citation summarizing the achievement and its significance; if personal name(s) are included, such name(s) must follow the achievement itself in the citation wording: Text absolutely limited by plaque dimensions to 70 words; 60 is preferable for aesthetic reasons.
The first atomic clock, developed near this site by Harold Lyons at the National Bureau of Standards, revolutionized timekeeping by using transitions of the ammonia molecule as its source of frequency. Far more accurate than previous clocks, atomic clocks quickly replaced the Earth’s rotational rate as the reference for world time. Atomic clock accuracy made possible many new technologies, including the Global Positioning System (GPS).
200-250 word abstract describing the significance of the technical achievement being proposed, the person(s) involved, historical context, humanitarian and social impact, as well as any possible controversies the advocate might need to review.
IEEE technical societies and technical councils within whose fields of interest the Milestone proposal resides.
In what IEEE section(s) does it reside?
Washington Section (Region 2)
IEEE Organizational Unit(s) which have agreed to sponsor the Milestone:
IEEE Organizational Unit(s) paying for milestone plaque(s):
Unit: UFFC Society
Senior Officer Name: John Vig, former IEEE President
Unit: UFFC Society
Senior Officer Name: Clark Nguyen, UFFC president
Unit: IEEE-USA
Senior Officer Name: Peter Eckstein
IEEE Organizational Unit(s) arranging the dedication ceremony:
Unit: IEEE USA through Washington Section
Senior Officer Name: Tony Ivanov
Unit: UFFC Society
Senior Officer Name: Clark Nguyen, UFFC president
IEEE section(s) monitoring the plaque(s):
IEEE Section: Washington DC
IEEE Section Chair name: Tony Ivanov
Milestone proposer(s):
Proposer name: Michael Lombardi
Proposer email: Proposer's email masked to public
Proposer name: Tom O'Brian
Proposer email: Proposer's email masked to public
Please note: your email address and contact information will be masked on the website for privacy reasons. Only IEEE History Center Staff will be able to view the email address.
Street address(es) and GPS coordinates in decimal form of the intended milestone plaque site(s):
There are two possible sites where the plaque can be located. Site 1 is located the closest to where the original Radio Building was located (see text below regarding original building site) , Site 2 would be seen more by the public and is also very near the site of the original work. The sites are described below:
Site 1 - International Drive NW, Washington, DC (across the street from Embassy of Kingdom of Bahrain)
There is a park owned by the U. S. State Department that is surrounded by various embassies. There is a retaining wall with a staircase into the park that would be a suitable location for the plaque. This is the nearest possible location to where the atomic clock was developed. A photograph (from Google Maps) is shown below. The coordinates are 38.941789, -77.067132.
Site 2 - 4000 Connecticut Avenue NW, Washington, DC
One remaining piece of the old National Bureau of Standards (NBS) campus remains, a pair of stone columns that once served as an entrance to the NBS campus (see photograph below from Google Maps, the two columns are circled in the photo). This site is slightly further away from where the work was accomplished than Site 1, but is on a major road where a steady stream of people would walk by and see the plaque. The columns make up the border of another little park, also owned by the U. S. State Department.
Describe briefly the intended site(s) of the milestone plaque(s). The intended site(s) must have a direct connection with the achievement (e.g. where developed, invented, tested, demonstrated, installed, or operated, etc.). A museum where a device or example of the technology is displayed, or the university where the inventor studied, are not, in themselves, sufficient connection for a milestone plaque.
Please give the details of the mounting, i.e. on the outside of the building, in the ground floor entrance hall, on a plinth on the grounds, etc. If visitors to the plaque site will need to go through security, or make an appointment, please give the contact information visitors will need. See descriptions and photographs of Site 1 and Site 2 above.
There is a historical marker located inside the retaining wall of Site 1 denoting the "Newton Apple Tree" that was planted in the park by NIST in 2000. A photograph of this plaque is shown below.
Are the original buildings extant?
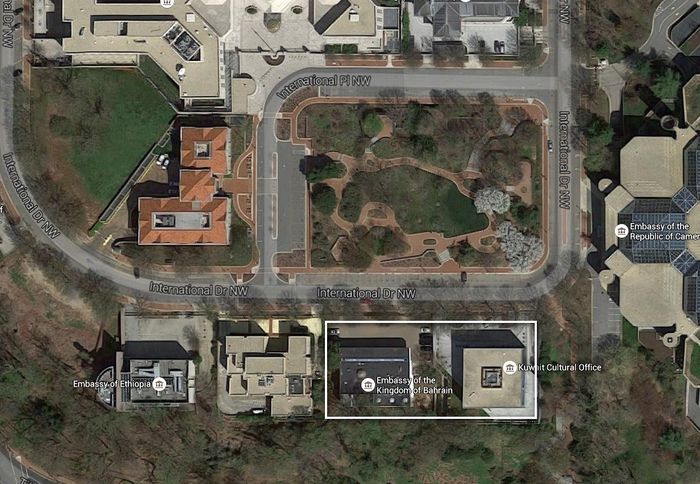
No. The Bureau of Standards relocated from Washington, DC to Gaithersburg, Maryland beginning in 1962 and ending in 1968. Lyons and his group worked in the Radio Building on the old DC campus and all of the buildings on that campus have been torn down. Based on comparisons of aerial photos of the DC campus with current images from Google maps, the approximate location of the Radio Building was where the embassies of Bahrain and the Kuwait Cultural Office are now located. The Bahrain embassy is located at 3502 International Drive NW in Washington, DC, and the Kuwait Cultural Office is located at 3500 International Drive NW. Here is an aerial view from Google Maps:
Here is a photograph of the Radio Building (probably taken in the 1920s):
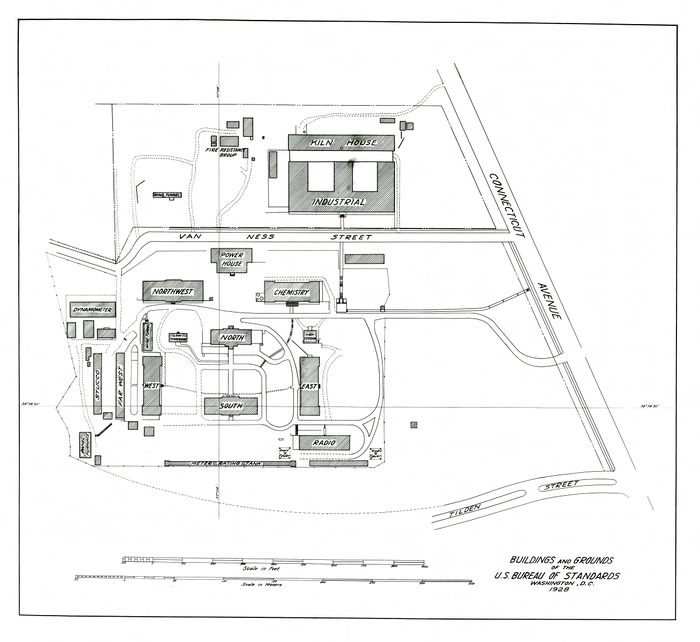
Here is a 1928 map of the NBS campus showing where the radio building was located with respect to Connecticut Avenue and Tilden Street:
Details of the plaque mounting:
The plaque will be mounted outdoors, either on a stone retaining wall or a stone column (see description and photographs of Site 1 and Site 2 above).
How is the site protected/secured, and in what ways is it accessible to the public?
Both of the proposed sites are publicly accessible in an outdoor location. The area is regularly patrolled by security/police officers.
A duplicate copy of the plaque will be mounted in the lobby of the Administration Building at the National Institute of Standards and Technology in Gaithersburg, Maryland (100 Bureau Drive, Gaithersburg, MD, 20899). This site is approximately 18 miles (29 km) away from the site of invention by car, but will allow NIST employees and visitors who pass through security to view the plaque. NIST visitors can normally pass through security if sponsored by a NIST employee.
Who is the present owner of the site(s)?
The United States State Department
What is the historical significance of the work (its technological, scientific, or social importance)? If personal names are included in citation, include detailed support at the end of this section preceded by "Justification for Inclusion of Name(s)". (see section 6 of Milestone Guidelines)
The societal impact of atomic clocks has been immense. Many technologies that we take for granted rely on atomic clock accuracy, including mobile phones, Global Positioning System (GPS) satellite receivers, and the electric power grid.
Atomic clocks fundamentally altered the way that time is measured and kept. Before atomic clocks, the second was defined by dividing astronomical events, such as the solar day or the tropical year, into smaller parts. This changed in 1967, when the second was redefined as the duration of 9 192 631 770 energy transitions of the cesium atom. The new definition meant that seconds were now measured by counting oscillations of atoms, and minutes and hours were now multiples of the second rather than divisions of the day.
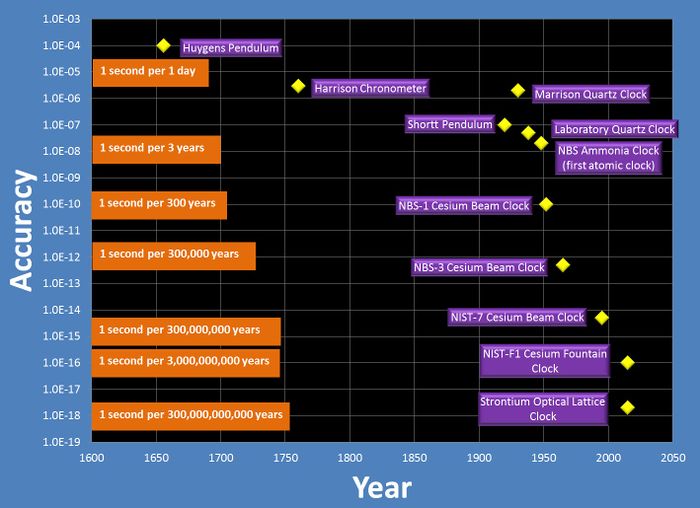
Atomic clocks also enabled astounding gains in timekeeping accuracy. For thousands of years prior to the invention of atomic clocks, the reference for world timekeeping was the Earth’s rotation rate, which was limited in accuracy to about one millisecond (one part in 10^8) per day. Quartz oscillators first appeared in the 1920s. The best quartz devices were eventually accurate enough to measure and record variations in the Earth’s rotation, but they were still limited in performance and sensitive to environmental changes. Atomic clocks provided a “quantum leap” in accuracy. Shortly after their invention, accuracies of parts in 10^10 became routine, and the accuracy of atomic clocks constructed at NBS and its successor, the National Institute of Standards and Technology (NIST), has increased by roughly one order of magnitude per decade as shown in the illustration below.
The Scottish physicist James Clerk Maxwell was perhaps the first to recognize that atoms could be used to keep time. In an era where the Earth’s rotation was the timekeeping standard, Maxwell remarkably suggested to William Thomson (Lord Kelvin) that the “period of vibration of a piece of quartz crystal” would be a better absolute standard of time than the mean solar second, but would still depend “essentially on one particular piece of matter, and is therefore liable to accidents.” Atoms would work even better as a natural standard of time. Thomson wrote in the second edition of the Elements of Natural Philosophy, published in 1879, that atoms such as hydrogen or sodium are “absolutely alike in every physical property” and “probably remain the same so long as the particle itself exists.”
Even so, atomic clock experiments didn’t begin until about sixty years after the suggestions of Maxwell and Thomson. The early experiments were finally made possible by the rapid advances in quantum mechanics and microwave electronics that took place before, during, and after World War II. Most of the concepts that led to atomic clocks were developed by Isidor Isaac Rabi and his colleagues at Columbia University in the 1930’s and 40’s. As early as 1939, Rabi had informally discussed applying his molecular beam magnetic resonance technique as a time standard with scientists at NBS. Rabi and his colleagues at Columbia first measured the cesium resonance frequency in 1940, estimating the frequency of the hyperfine transition as 9191.4 megacycles. This was relatively close to the number that would later define the second. Rabi’s work led to a Nobel prize in 1944, but atomic clock research at Columbia was mostly halted during World War II as scientists turned their attention to other areas and a demonstrable device was not built.
Building on the accomplishments of previous researchers, Harold Lyons and his colleagues at NBS in Washington, DC began working towards the development of the first atomic clock in 1947. The NBS clock was based not on cesium atoms, but instead used the 23.8 GHz inversion transition of the ammonia molecule as its source of frequency. After a period of intense and rapid research and development beginning in the spring of 1948, the new clock was first operated on August 12, 1948 and was publicly demonstrated in January 1949. Although its accuracy was quickly surpassed by other atomic clocks, it marked the beginning of the era of atomic timekeeping.
Photographs of first atomic clock:
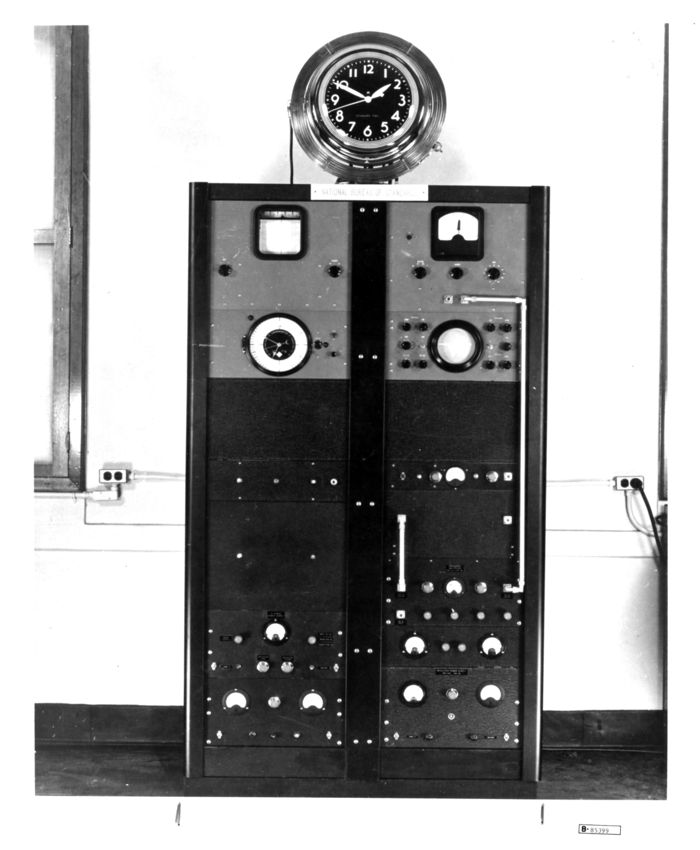
The world's first atomic clock as it appeared on January 6, 1949, the day of the first public announcement. An ammonia absorption cell consisting of about 8 meters of K-band waveguide is coiled around the round electric clock dial. The clock atop the equipment racks was driven by a 50 Hz signal obtained by dividing the signal from a quartz oscillator that was locked to the ammonia absorption frequency. However, its primary function was to let the world know that the new invention was indeed a clock.
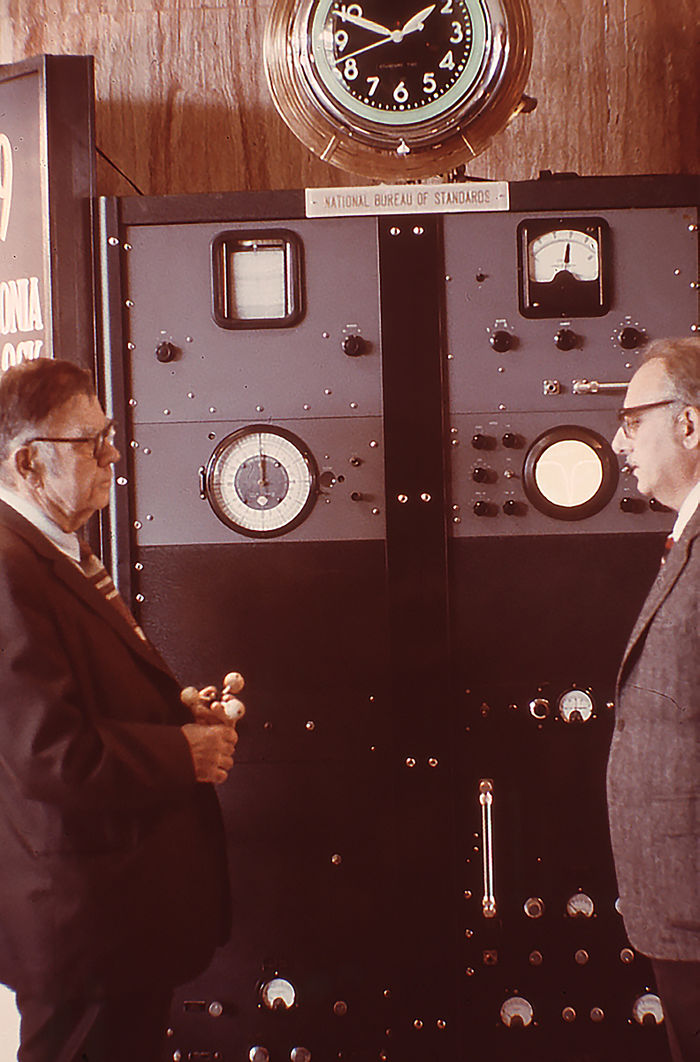
Dr. Harold Lyons (right), inventor of the ammonia absorption cell atomic clock, observes, while Dr. Edward U. Condon, the director of the National Bureau of Standards, examines a model of the ammonia molecule (1949).
A recreation of the original photo taken on the 25th anniversary of the atomic clock's invention. Dr. Harold Lyons (right), inventor of the ammonia absorption cell atomic clock, observes, while Dr. Edward U. Condon, the former director of the National Bureau of Standards, examines a model of the ammonia molecule (1974).
A 1948 photograph showing the backside of the first atomic clock and the electronic components contained inside the two equipment racks. Lyons is kneeling on the right.
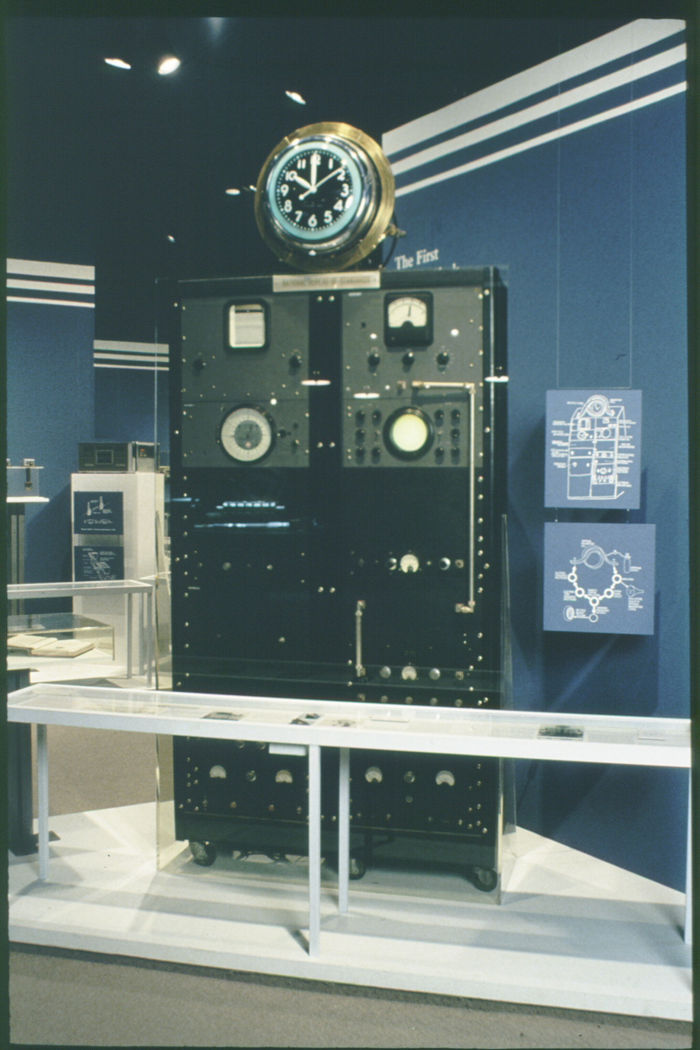
The Division of Work and Industry of the Smithsonian Institute's National Museum of American History, holds the first atomic clock, constructed under Lyons’s direction at the National Bureau of Standards in 1948. The photograph shows the clock while on display at the museum.
What obstacles (technical, political, geographic) needed to be overcome?
The basic principles of atomic clocks were already well understood by researchers at the time of Lyon’s invention. In short, because all atoms or molecules (groups of atoms held together by a chemical bond) of a specific element are identical, they should produce the exact same frequency when they absorb energy or release energy. Thus, an atom or molecule could potentially serve as a “perfect” oscillator, and a clock referenced to an atomic oscillator would be far more accurate than previous clocks based on mechanical oscillations of a pendulum or a quartz crystal.
Even though the potential advantages of atomic timekeeping were well known, many obstacles existed before an atomic clock could be built. Atomic transitions occurred in the microwave region, at much higher frequencies than had previously been measured, and systems and electronics were needed that could measure these frequencies by counting the atomic transitions. Determining the resonance frequency of a specific element was necessary so that it could be related to the second, the base unit of time interval. The next step would be to use the atomic resonance frequency to control the frequency of a physical oscillator, such as quartz crystal oscillator, by locking it to atomic resonance. Finally, to make the output of an atomic clock usable, the frequency of the locked quartz oscillator would need to be divided to frequencies low enough to utilize for keeping time.
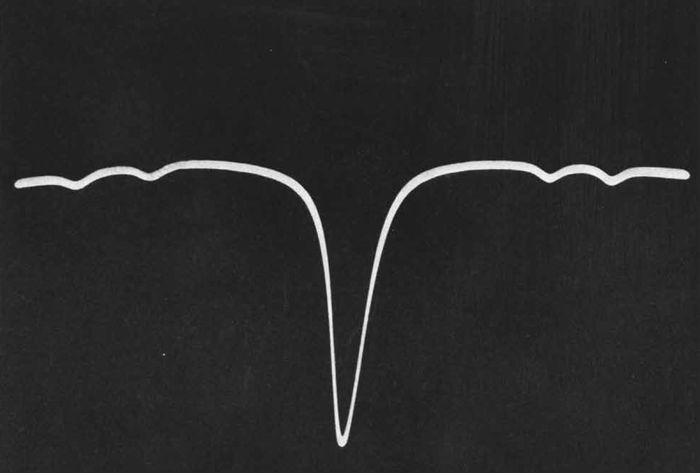
Lyons had chosen the absorption line of ammonia gas as the source of frequency for the first atomic clock, and his first obstacle to overcome was measuring the inversion transition frequency of the ammonia molecules. The ammonia gas was stored in an absorption cell, which was designed as a 30 foot long copper tube that was wrapped into a spiral. The measurement involved multiplying a signal from a 100 kHz quartz oscillator several times. The first stage consisted of a frequency multiplication chain built from low frequency tubes that resulted in a frequency of about 270 MHz. The second stage consisted of a frequency multiplying klystron (a linear beam vacuum tube), frequency modulated by a 13.8 MHz oscillator that multiplied the signal to about 2.983 GHz. Finally, the signal was multiplied to approximately 23.87 GHz by use of a silicon crystal rectifier. This frequency was fed to the ammonia absorption cell and tuned until the signal reaching a silicon crystal detector at the end of the cell dipped because of the absorption, as shown in the illustration. This dip indicated that the incoming frequency from the multiplication chain now matched the ammonia absorption frequency. When the “dip” occurred, an electrical pulse was generated.
The "dip" (shown on an oscilloscope) that occurred when the incoming microwave signal from the frequency multiplication chain matched the absorption frequency of the ammonia molecule.
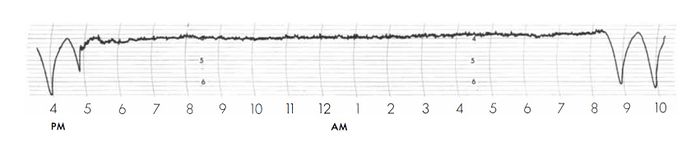
The next obstacle faced by Lyons and his group was developing a method to automatically adjust the quartz oscillator frequency until it matched the frequency of ammonia absorption line. This was done by taking a 12.5 MHz frequency from the quartz oscillator frequency multiplication chain and mixing it with the signal from the 13.8 MHz frequency-modulated oscillator. When the frequency from the mixer matched its expected value, another electrical pulse was generated. The time interval between the two generated pulses (the pulse from the absorption cell and the pulse from the mixer) was continuously measured. If the time interval was increasing it indicated that quartz oscillator frequency was increasing, and vice versa. The time interval measurements were used to generate a control signal that adjusted the quartz oscillator whenever necessary to keep it locked to the ammonia absorption frequency. The illustration shows a strip chart where the quartz oscillator was locked to the ammonia absorption frequency from 5 p.m. to 8 a.m. Before and after this period, the quartz oscillator was “unlocked” (free running). Each small division on the strip chart represents a frequency variation of less than 1 part per 10 million.
A strip chart showing a period (from 5 p.m. to 8 a.m.) where the quartz oscillator was locked to the absorption frequency of ammonia.
Another obstacle in building the atomic clock consisted of dividing the locked quartz oscillator frequency to low frequencies that could be used for timekeeping. The first atomic clock included dividers that produced two output signals, a 50 Hz signal that drove an ordinary synchronous motor clock, and also a 1000 Hz signal that was used to drive a special synchronous motor clock that was designed for comparisons with astronomical time (then the world standard for timekeeping) to within 5 milliseconds. The two block diagrams show the various components and functions of the first atomic clock, first in simplified form, and then in more detail.
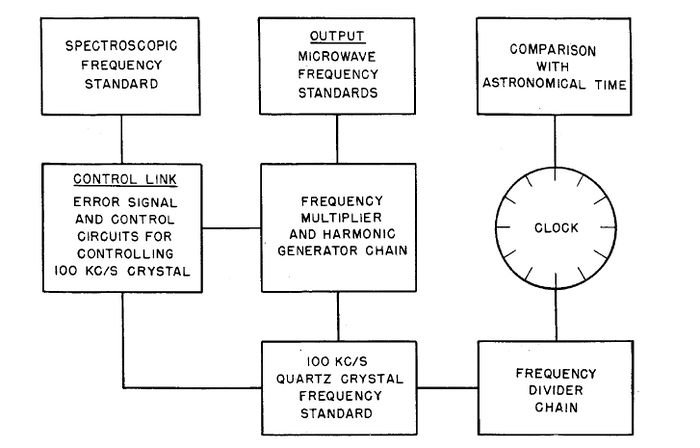
A simplified block diagram of the first atomic clock.
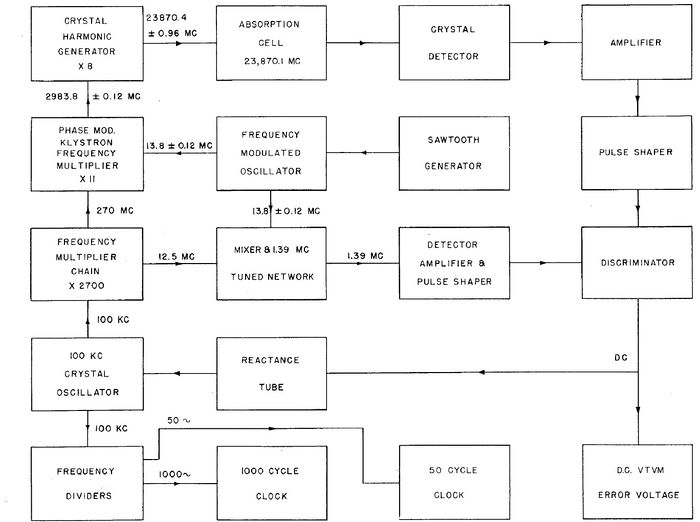
A complete block diagram of the first atomic clock.
A final technical obstacle faced by Lyons was his quest to make his ammonia clock more accurate by increasing the quality factor, Q, which is the resonance frequency of an oscillator divided by the resonance width (or linewidth). Oscillators with higher Qs are potentially more stable and accurate, so it is desirable to increase Q as much as possible, either by using an atomic transition where the frequency is as high as possible, or by making the line width as narrow as possible. In the case of the ammonia clock, the resonance frequency was high (23.8 GHz), but the resonance width, or the range of frequencies over which the oscillator could resonate (represented by the dip in the absorption frequency shown above) was not particularly narrow. Thus, the potential accuracy of the ammonia clock was limited. Two versions of the ammonia clock were built, with the best reported accuracy about 2 ms per day (2 × 10-^8). Work on a third version was halted when it became apparent that cesium beam techniques were likely to provide a significant increase in accuracy. Even though the cesium resonance was not as high (9.192 GHz) there were numerous options available for decreasing the resonance width, leading to Q factors that were potentially much higher than those obtained with molecular absorption methods. As a result Lyons and his NBS colleagues turned their attention from ammonia to cesium clocks in 1950.
What features set this work apart from similar achievements?
Lyons was certainly not the first to perform spectroscopic frequency stabilization experiments or to engage in atomic clock research. He was just 35 years old at the time he first demonstrated the ammonia clock to the public in early 1949, and was a 25-year old graduate student when Rabi and his colleagues at Colombia first discussed employing their molecular beam resonance technique as a time standard. However, Lyons and his colleagues were able to rapidly move past the pure experimental stage and become the first to build a working atomic clock.
The construction of an atomic clock in 1948 was a monumental engineering feat. In addition to the ammonia absorption cell, the new invention consisted of a number of electronic instruments including a quartz crystal oscillator, several stages of frequency multipliers, a frequency discriminator, and frequency dividers, integrated into a single system and housed in two large equipment racks. These instruments, along with the expertise obtained by NBS in several decades of maintaining and dissemination standard frequency signals, allowed Lyons to advance beyond what had previously achieved elsewhere. He was able to excite an ammonia absorption cell with the output of a frequency multiplier chain that could be measured against the national standard of frequency, quartz oscillators operated by NBS that were periodically calibrated against the astronomical time standards maintained by the U. S. Naval Observatory. This direct access to the best available time standards allowed Lyons to have confidence in his results and to verify that his new invention was working properly.
Why was the achievement successful and impactful?
Supporting texts and citations to establish the dates, location, and importance of the achievement: Minimum of five (5), but as many as needed to support the milestone, such as patents, contemporary newspaper articles, journal articles, or chapters in scholarly books. 'Scholarly' is defined as peer-reviewed, with references, and published. You must supply the texts or excerpts themselves, not just the references. At least one of the references must be from a scholarly book or journal article. All supporting materials must be in English, or accompanied by an English translation.
Patents:
[P1] H. Lyons and B. F. Huston, "Atomic Clock," United States Patent 2,699,503 (Applied for April 30, 1949, granted January 11, 1955).
Journal Articles:
[J1] H. Lyons, "The Atomic Clock," Instruments, vol. 22, pp. 133-135, 174, December 1949.
Media:Lyons_Instruments_Dec_1949.pdf
[J2] "The Atomic Clock: An Atomic Standard of Frequency and Time," Journal of the Horological Institute of America, published in two parts in 1949 (issue 2, pp. 11-20 and issue 3, pp. 7-14).
A copy of the original journal paper is not available but the text is identical to the following document, which is provided here:
National Bureau of Standards, "The Atomic Clock: An Atomic Standard of Frequency and Time," NBS Technical Report 1320, 27 pages, January 1949.
Media:NBS_Report_1320_1949.pdf
[J3] H. Lyons, “The Atomic Clock: A Universal Standard of Frequency and Time,” American Scholar, vol. 19, pp. 159-168, April 1950.
Media:Lyons_American_Scholar_1950.pdf
[J4] H. Lyons, "Microwave Frequency Dividers," Journal of Applied Physics, vol. 21, pp. 59-60, January 1950.
Media:Lyons_J_Applied_Physics_1950.pdf
[J5] H. Lyons, “Spectral lines as frequency standards,” Annals of the New York Academy of Sciences, vol. 55, pp. 831-871, November 1952.
Media:Lyons_New_York_Academy_1952.pdf
[J6] H. Lyons, "Atomic Clocks," Scientific American, vol. 197, pp. 71-82, February 1957.
Media:Lyons_Scientific_American_1957.pdf
[J7] R. Beehler, “A Historical Review of Atomic Frequency Standards,” Proceedings of the IEEE, vol. 55, pp. 792-805, June 1967.
Media:Beehler_Proc_IEEE_1967.pdf
[J8] P. Forman, “Atomichron: The Atomic Clock from Concept to Commercial Product,” Proceedings of the IEEE, vol. 73, pp. 1181-1204, July 1985.
Media:Forman_Proc_IEEE_1985.pdf
[J9] M. A. Lombardi, T. P. Heavner, and S. R. Jefferts, "NIST Primary Frequency Standards and the Realization of the SI Second," NCSLI Measure: The Journal of Measurement Science, vol. 2, no. 4, pp. 74-89, December 2007.
Media:Lombardi_Measure_2007.pdf
[J10] M. A. Lombardi, "The Evolution of Time Measurement, Part 3: Atomic Clocks," IEEE Instrumentation and Measurement Magazine, vol. 14, pp. 46-49, December 2011.
Media:Lombardi_IEEE_IM_Magazine_2011.pdf
Conference Papers:
[C1] H. Lyons, "Microwave spectroscopic frequency and time standards," Proceedings of the IXth General Assembly of URSI, paper no. 91, pp. 47-57, 1950.
[C2] P. Forman, “The first atomic clock program: NBS, 1947-1954,” Proceedings of the 1985 Precise Time and Time Interval Meeting (PTTI), pp. 1-17, 1985.
[C3] D. B. Sullivan, "Time and Frequency Measurement at NIST: The First 100 Years," Proceedings of the 2001 IEEE Frequency Control Symposium, pp. 4-17, 2001.
Media:Sullivan_IEEE_FCS_2001.pdf
Books:
[B1] W. Snyder and C. Bragaw, “Achievement in Radio: Seventy Years of Radio Science, Technology, Standards, and Measurement at the National Bureau of Standards,” National Bureau of Standards Special Publication 555, October 1986.
Media:Snyder_NBS_SP_555_1986.pdf
[B2] C. H. Townes, "How the Laser Happened: Adventures of a Scientist," Oxford University Press: New York, 1999.
[B3] T. Jones, "Splitting the Second: The Story of Atomic Time," Institute of Physics Publishing, Bristol and Philadelphia, 2000.
News Articles:
[N1] “The Atomic Clock: An Atomic Standard of Frequency and Time,” National Bureau of Standards Technical News Bulletin, vol. 33, no. 2, pp. 17-24, February 1949.
Media:NBS_Bulletin_February_1949.pdf
[N2] "Atomic Clock," Review of Scientific Instruments, vol. 20, no. 2, pp. 141-142, February 1949.
Media:RSI_News_Release_Feb_49.pdf
Miscellaneous:
Cartoon, "Ripley's Believe it or Not!," King Features Syndicate, 1953.
Supporting materials (supported formats: GIF, JPEG, PNG, PDF, DOC): All supporting materials must be in English, or if not in English, accompanied by an English translation. You must supply the texts or excerpts themselves, not just the references. For documents that are copyright-encumbered, or which you do not have rights to post, email the documents themselves to ieee-history@ieee.org. Please see the Milestone Program Guidelines for more information.
Media:2016-05-03 NIST Letter Support for Historic Plaque.pdf
Media:Ivanov_Letter_from_Washington_IEEE_Section.pdf
Please email a jpeg or PDF a letter in English, or with English translation, from the site owner(s) giving permission to place IEEE milestone plaque on the property, and a letter (or forwarded email) from the appropriate Section Chair supporting the Milestone application to ieee-history@ieee.org with the subject line "Attention: Milestone Administrator." Note that there are multiple texts of the letter depending on whether an IEEE organizational unit other than the section will be paying for the plaque(s).
Please recommend reviewers by emailing their names and email addresses to ieee-history@ieee.org. Please include the docket number and brief title of your proposal in the subject line of all emails.